Textile Dyeing
Dyes are soluble coloured organic compounds that are usually applied to textiles from a solution in water. They are designed to bond strongly to the polymer molecules that make up the textile fibre. A dye in solution is coloured because of the selective absorption of certain wavelengths of light by specific bonds in the molecule. The light that is transmitted is seen by the observer and appears coloured because some of the wavelengths of the visible spectrum are now missing (absorbed).
While there are number of dyes available in the market, a good understanding
1. Classification of Dyes
1.1 Based on Chemical structures
Based on chemical structures, there are around 25 different structural classes of dyes in use, the most important being:
1) Azo dyes:
Representing more than characterized by the presence of one or more azo groups (![]() ), together with hydroxyl groups, amine and substituted amine groups as auxochromes.
), together with hydroxyl groups, amine and substituted amine groups as auxochromes.
Azobenzene is the chromophore of these azo dyes.
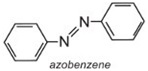
The colour of the molecule can be modified and the intensity of colour increased by varying the auxochromes (as in the table below).
| Structure | Colour observed |
|---|---|
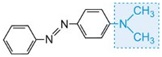 | yellow-green |
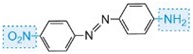 | yellow |
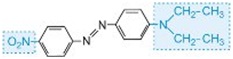 | red |
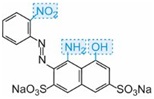 | blue |
Table: The molecular structures of some azo dyes showing the auxochromes
2) Anthraquinone dyes:
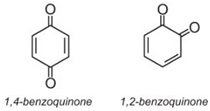
Anthraquinone dyes account for about 15% of colorants and have structures based on quinones, e.g. benzoquinone, anthraquinones (which are, in turn, based on anthracene), etc
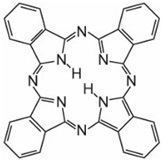
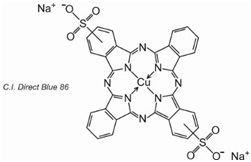
3) Phthalocyanines: made up of four molecules of isoindole (![]() ), the final structure coordinating with metal atoms, The most important, contributing about 2% of all colorants, are the copper phthalocyanines, used for their brilliant blue and green colours, e.g. C.I. Direct Blue 86
), the final structure coordinating with metal atoms, The most important, contributing about 2% of all colorants, are the copper phthalocyanines, used for their brilliant blue and green colours, e.g. C.I. Direct Blue 86
1.2 Based on source:
Dyes used in textile industry are broadly categorized based on the source, from which they are obtained, as:
- Natural Dyes
- Synthetic Dyes
1.3 Industrial Classification:
Classified based upon their chemical composition and the method of their application in the dyeing process, textile dyes include:
- Acid dyes, used mainly for dyeing wool, silk, and nylon. A typical recipe calls for dissolving dye and salt in adequate water to cover the material, heat it to a simmer for ten minutes, add vinegar (making it acidic) and simmer for another ten minutes, then allow to cool gradually and rinse out. Unlike the vat and sulphur dyes, they are water-soluble and can be applied directly to the fiber.
- Azoic dyes are insoluble pigments formed within the fibers. These dyes cannot be applied directly on the fibers as dyes. For this production, first the fiber is impregnated with soluble coupling component and then with a diazotized base, forming the azo dyes within the fibers.
- Basic (Cationic) Dyes are water-soluble and are mainly used to dye acrylic fibers. They are mostly used with a mordant. A mordant is a chemical agent which is used to set dyes on fabrics by forming an insoluble compound with the dye. With mordant, basic dyes are used for cotton, linen, acetate, nylon, polyesters, acrylics and modacrylics. Other than acrylic, basic dyes are not very suitable for any other fiber as they are not fast to light, washing or perspiration. Thus, they are generally used for giving an after treatment to the fabrics that have already been dyed with acid dyes.
- Direct (substantive) Dyes color cellulose fibers directly without the use of mordants. They are used for dyeing wool, silk, nylon, cotton, rayon etc. These dyes are not very bright and have poor fastness to washing although they are fairly fast to light.
- Mordant Dyes (or chrome dyes) are acidic in character. Sodium or potassium bichromate is used with them in the dyebath or after the process of dyeing is completed. This is done for getting the binding action of the chrome. They are mostly used for wool which gets a good color fastness after treatment with mordant dyes. They are also used for cotton, linen, silk, rayon and nylon (but are less effective for them).
- Vat dyes are insoluble in water and cannot dye fibers directly. However, they can be made soluble by reduction in alkaline sodium hydrosulphite which allows them to affix to the textile fibers. Cellulose absorbs these colourless compounds, which are subsequently oxidized to an insoluble pigment. Subsequent oxidation or exposure to air restore the dye to its insoluble form. Indigo is the original vat dye. These dyes are the fastest dyes for cotton, linen and rayon. They are used with mordants to dye other fabrics such as wool, nylon, polyesters, acrylics and modacrylics.
- Reactive Dyes react with fiber molecules to form a chemical compound, resulting in excellent colourfastness. These dyes are either applied from alkaline solution or from neutral solutions which are then alkalized in a separate process. Sometimes heat treatment is also used for developing different shades. After dyeing, the fabric is washed well with soap so as to remove any unfixed dye. Reactive dyes were originally used for cellulose fibers only but now their various types are used for wool, silk, nylon, acrylics and their blends as well.
- Disperse Dyes are water insoluble. These dyes are finely ground and are available as a paste or a powder that gets dispersed in water. These particles dissolve in the fibers and impart color to them. These dyes were originally developed for the dyeing of cellulose acetate but now they are used to dye polyester, nylon, cellulose triacetate, and acrylic fibers too.
- Sulphur Dyes are insoluble and made soluble by the help of caustic soda and sodium sulphide. Dyeing is done at high temperature with large quantities of salt so that the color penetrates into the fiber. After dyeing the fabric is oxidized for getting desired shades by exposure to air or by using chemicals. Excess dyes and chemicals are removed by thorough washing. These dyes are fast to light, washing and perspiration and are mostly used for cellulosic fabrics, e.g. cotton and linen.
- Although Pigment dyes are not dyes in a true sense (dyes are soluble while pigments are not expected to be soluble), they are extensively used for colouring fabrics like cotton, wool and other manmade fibers due to their excellent light fastness. They do not have any affinity to the fibers and are affixed to the fabric with the help of resins. After dyeing, the fabrics are subjected to high temperatures.
2. Fabric types and compatible dyes
There are different ways of dye applications based on the dye type and the fabric material. The basic features that control dye transfer from solution to fabric material are:
- The pH of the solution in the dyebath (for acid and basic dyes)
- An electrolyte (a solution of sodium sulphate or chloride)
- The temperature (within the range of ambient to 400 K)
- Chemicals, known as dispersing agents that produce a stable aqueous dispersion of dyes of very low solubility
The below table lists the dyes under their technological names that indicate how they are applied, along with the fibres to which they are applied:
| Dye | Fiber |
|---|---|
| Acid | Wool and other protein fibres, polyamides |
| Metal-complex | Wool and other protein fibres, polyamides |
| Direct | Cotton, linen, viscose |
| Basic | Acrylic |
| Disperse | Polyesters, polyamides, ethanoates |
| Reactive | Cotton, linen, viscose, wool, silk |
| Vat | Cotton, linen, viscose |
| Sulphur | Cotton, linen |
On the other hand below table lists out the dyes suitable for each fiber material:
| Fibres | Dye Application Classes |
|---|---|
| Wool | Acid, basic, mordant, reactive, (solubilized vat) |
| Wool blends | Acid, direct, mordant, reactive |
| Silk | Acid, basic, direct, mordant, (reactive), (solubilized vat) |
| Cotton | Azoic, basic, direct, mordant, oxidation, reactive, Sulphur, vat |
| Viscose | Direct, mordant, pigment, reactive, sulphur, vat, solubilized vat |
| Polyamide | Acid, disperse, mordant, pigment, reactive |
| Polyester | Disperse, pigment |
| Polyacrylonitrile | Basic, disperse, pigment |
| Polyvinyl chloride | Basic, disperse |
| Polyolefin | Disperse |
| Elastomers | Acid, disperse, reactive, (wool), vat |
3. Environmental Concerns
The contamination of natural waters has become one of the biggest problems in modern society, and the economical use of this natural resource in production processes has gained special attention. Textile industry has contributed majorly to these problems mainly due to the volumes and complexity. In general, effluents from the textile industry are extremely complex, since they contain a large variety of dyes, additives and derivatives that change seasonally, increasing the challenge to find effective, feasible treatments.
Of the industries with high-polluting power, the textile industry, dyeing being the biggest factor, stands out. Textile dying has been a major source of concern owing to the pollution, colouration, toxicity and other harmful effects on water bodies. The difficulty in removing textile dyes from the environment can be attributed to the high stability of these compounds, since they are designed to resist biodegradation to meet the demands of the consumer market with respect to durability of the colors in the fibers, consequently implying that they also remain in the environment for a long time. The processes are being used that are not entirely appropriate for the treatment of textile effluents, thereby creating a major challenge for the industry and laundries that need to adapt to current regulations for the control of the color of effluents with a high organic load.
Many federal countries have national environmental legislation, while many have just established the limits that must be complied with, some countries have copied the other models, while in some countries, the emission limits are recommended, but are not mandatory.
4. The way forward
4.1 Water Usage: Waterless dyeing and less-water dyeing methods are leading the way in pursuit to tackle the menace of water pollution created by textile dyeing.
Reuse of water effluent is another wise option. Many textile players in the textile industry are making attempts to elevate the water quality of wastewater effluent from a secondary wastewater treatment plant to a higher standard for reuse.
4.2 Effective Dying: An alternative to minimize the problems related to the treatment of textile effluents would be to develop more effective dye that can be fixed on fibers with higher efficiency decreasing losses on tailings waters and reducing the amount of dye required in the dyeing process, certainly improving the quality of the effluent.
4.3 Solution Dyed yarns: Dope dyeing or solution dyeing has arrived as a practical solution to get rid of water requirement in textile dyeing process. Here the dye is added in the master-batch for the synthetic yarns and the yarn is dyed to very high level of colour fastness. The industry has been able to get rid of the bottleneck of minimums leading to greater demands and adaptation of solution dyed yarns
5. Conclusion
Synthetic textile dyes represent a large group of organic compounds that could have undesirable effects on the environment, and in addition, some of them can pose risks to humans. The increasing complexity and difficulty in treating textile wastes has led to a constant search for new methods that are effective and economically viable. However, up to the present moment, no efficient method capable of removing both the color and the toxic properties of the dyes released into the environment has been found. Various alternatives are being worked out to reduce or eliminate the need for water in dyeing process with the broader vision to minimize the impact of textile dyeing on the ecosystem.
Share This Post